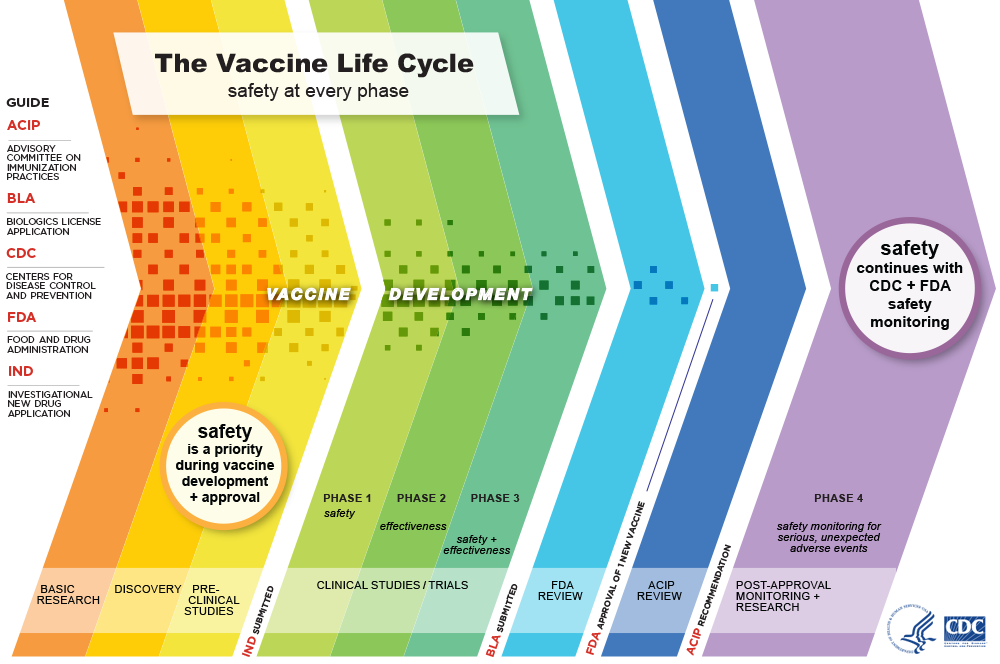
Through decades of research, testing and development, vaccines have proven to be an effective way to prevent many infectious diseases and their effects, including serious illness, hospitalization and death. Vaccines are held to a high safety standard, pass lots of testing and are constantly monitored for safety throughout all stages of use.
Most people do not experience serious side effects from vaccines. Some mild side effects can sometimes occur, such as pain or swelling at the injection site, and these side effects are often signs that a body is building a healthy immune response and protection well.
Getting multiple vaccinations at once has been shown to be safe.
Vaccine Adverse Event Reporting System (VAERS)
It is rare, but an adverse event (possible side effect) can occur after a person receives a vaccine dose. The cause of these adverse events can be difficult to identify and are often from an unrelated cause, not the vaccine. Scientists monitor for immunization response trends and unusual patterns of adverse events. Vaccine Adverse Event Reporting System (VAERS) is used by the Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) to collect reports of side effects that happen after vaccination. The system relies on individuals to report adverse health events following vaccination – anyone can and should report adverse events to VAERS (hhs.gov).
How to Report an Adverse Event to VAERS
Created in 1990 by the US Department of Health and Human Services, VAERS is a database for the collection of adverse events following vaccines. It is operated and monitored by the CDC and the FDA to detect whether any vaccines are associated with higher than expected rates of adverse events.
Anyone can report to VAERS. This can be vaccine recipients themselves, caregivers of vaccine recipients (parents, guardians) or health care providers. Any clinically significant or unexpected events following a vaccine administration should be reported to VAERS. See VAERS Table of Reportable Events Following Vaccination (PDF).
Adverse events that follow vaccines are rare. Most adverse events that follow vaccines are manageable by health care providers that administer vaccines and will occur within hours of administration. Some extremely rare events can occur within six months of vaccine administration. People with conditions known to make them more vulnerable to these events should talk to their health care provider before receiving a vaccine. Some adverse events and their expected timeline after vaccination are listed here: Vaccine Injury Table Effective for Claims Filed on or After 1-3-2022 (hrsa.gov).
North Dakota Health and Human Services (NDHHS) does not maintain the VAERS database but does regularly review reports made by or on behalf of North Dakotans. To contact the NDHHS Immunization Unit for assistance with or questions about VAERS, please contact vaccine@nd.gov.
How is Vaccine Safety Monitored in the U.S.?
The success of vaccine programs depends on vaccine effectiveness and safety. Because vaccines are given to millions of healthy people each year, they are held to a very high standard and are continuously monitored for safety. The U.S. has one of the most advanced systems in the world for assessing vaccine safety. This includes using state-of-the-art technologies and systems working together. Each of the systems supplies a different type of data for researchers to analyze. Together, they work as a well-oiled machine to help provide a comprehensive picture of vaccine safety in the U.S.
Safety Information by Vaccine
Additional Resources
- Common Vaccine Safety Questions and Concerns
- Certificate of Immunization and Exemption
- Vaccines During Pregnancy FAQs
- Vaccine Safety Datalink (VSD)
- Vaccine Safety Information for Parents and Caregivers
- Vaccine Safety Information for Health Care Providers
- Emergency Preparedness and Vaccine Safety
- Common Questions About Vaccines
- Vaccine Education Center at Children's Hospital of Philadelphia
Questions? Contact Us!
NDHHS Disease Control — Immunization Unit
600 E. Boulevard Avenue, Dept. 325
Bismarck, ND 58505-0200
Phone: (701) 328-3386
Toll-Free: (800) 472-2180
TTY: 711
Email: vaccine@nd.gov